Using bentonite-polymer composite geosynthetic clay liners to contain CCR leachates

Using bentonite-polymer composite geosynthetic clay liners to contain CCR leachates
- 0
Coal combustion residuals (CCRs) are the byproducts from combustion in coal-fired boilers that are disposed in lined landfills when they cannot be used beneficially in other applications (EPRI 2009) (Figure 1). Land disposal of CCRs is regulated under the “coal ash rule” incorporated into Subtitle D of the Resource Conservation and Recovery Act (RCRA) (Federal Register 2015). The coal ash rule requires that coal ash disposal facilities be lined with a composite liner consisting of a geomembrane underlain by a compacted clay liner at least 2 feet (0.6 m) thick having a hydraulic conductivity no greater than 1 × 10-9 m/s (Bittner et al. 2019). Geosynthetic clay liners (GCLs) can be used in lieu of compacted clay liners provided that the GCL meets the equivalency criteria in the coal ash rule. This generally requires that the hydraulic conductivity of the GCL be less than 3 × 10-11 m/s when tested with the CCR leachate to be contained (Bittner et al. 2019).
CCR leachates contain a variety of cations and anions that can affect the hydraulic conductivity of the GCL (Chen et al. 2018). Jo et al. (2001), Kolstad et al. (2004) and Xu et al. (2009) evaluated how ionic strength, cation valence and pH of permeant solutions affect the swelling and hydraulic conductivity of sodium bentonite (NaB) GCLs. They show that the hydraulic conductivity of NaB GCLs increase as the ionic strength of the leachate increases, and that GCLs are more permeable to solutions with polyvalent cations (e.g., calcium, magnesium, aluminum) than those with monovalent cations (e.g., sodium, potassium, lithium), all other factors being equal. Extreme pH (pH>13 or pH<2) also alters the hydraulic conductivity of NaB GCLs. Kolstad et al. (2004) evaluated how multispecies solutions affect swelling and hydraulic conductivity of NaB GCLs. They report that the ionic strength and the relative abundance of monovalent and polyvalent cations of a solution are master variables affecting swelling and hydraulic conductivity of NaB GCLs. Chen et al. (2018) evaluated the hydraulic conductivity of NaB GCLs permeated with CCR leachates representing a broad range of conditions in the U.S. They found that the hydraulic conductivity of NaB GCLs increased from 10-10 to 10-6 m/s as the ionic strength of the CCR leachate increased from 40 to 755 mM. Thus, for many CCRs, a conventional NaB GCL may not satisfy the criteria in the coal ash rule as an alternative to a compacted clay liner. In contrast to an NaB GCL, the hydraulic conductivity of most compacted clay liners is relatively insensitive to CCR leachates (Benson et al. 2018).
The chemical compatibility of GCLs has been enhanced in some applications by adding polymers to the bentonite (Di Emedio et al. 2011; Mazzieri et al. 2010; Scalia et al. 2014; Katsumi et al. 2008). These polymeric additions may be interlayer substitution or polymer surface treatment of the montmorillonite fraction (e.g., a polymer-modified bentonite [PMB]) or consist of a dry mixture of bentonite and polymer granules to form a bentonite-polymer composite (BPC) material. Some polymer additions are effective, whereas others are not. For example, Shackelford et al. (2010) report that the hydraulic conductivity of a PMB GCL was nine to 21 times higher than that of an NaB GCL when permeated with the same solution. In contrast, Scalia et al. (2014) report that the hydraulic conductivity of a BPC GCL permeated to calcium chloride (CaCl2) solutions was up to four orders of magnitude less permeable than an NaB GCL prepared with the same bentonite and permeated with the same CaCl2 solutions.
The hydraulic conductivity of several BPC GCLs to CCR leachates was evaluated in this study as part of chemical compatibility testing conducted to identify suitable GCL products for CCR disposal facilities. Polymer loading was also measured to understand mechanisms affecting hydraulic conductivity of the BPC GCLs.
Materials CCR leachates
Seven CCR leachates were obtained from coal ash disposal facilities in Virginia (CCR-VA1, CCR-VA2, CCR-VA3 and CCR-VA4); Wyoming (CCR-WY); and Minnesota (CCR-MN1, CCR-MN2). Two of the synthetic CCR leachates (i.e., flue gas desulfurization [FGD] and high strength [HS]) from Chen et al. (2018) were also used. Bulk chemical parameters, including pH, electrical conductivity (EC), ionic strength (I), relative abundance of monovalent and polyvalent cations (RMD) and anion ratio (molar ratio of chloride to sulfate, Cl–/SO42-), are summarized in Table 1. The pH of the CCR leachates ranges from 4.3 to 9.9, the ionic strength ranges from 33 to 681 mM and the EC ranges from 0.3 to 4.4 S/m at 77°F (25°C). The leachates range from chloride rich (CCR-VA2) to sulfate rich (CCR-VA1).
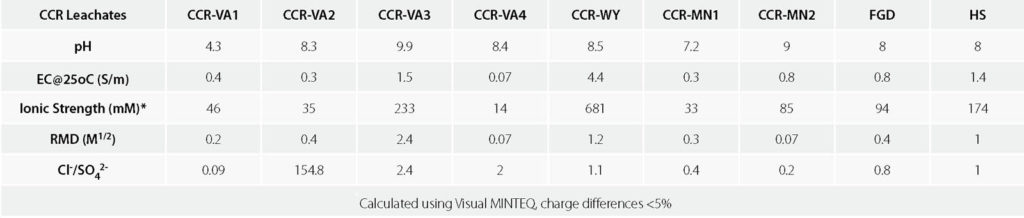
Kolstad et al. (2004) defined the parameter RMD to quantify the relative abundance of monovalent and polyvalent cations in a permeant liquid (Equation 1):
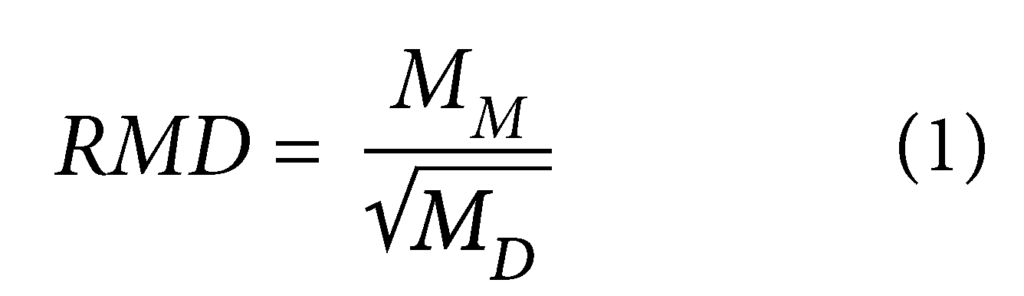
where MM is the total molarity of the monovalent cations and MD is the total molarity of polyvalent cations in the permeant solution. RMD of the CCR leachates ranges from 0.07 to 2.4 M1/2, or predominantly polyvalent (low RMD) to predominantly monovalent (high RMD).
The relationship between RMD and ionic strength for the CCR leachates is shown in Figure 2 along with leachates in the Electric Power Research Institute (EPRI) leachate database reported by Chen et al. (2018, 2019). The CCR leachates in this study (closed symbols in the figure) predominantly have ionic strengths in the upper portion of the ionic strengths in the EPRI leachate database and tend to have lower RMD (more polyvalent).

0 Comments